The fuel of rocket
The fuel of 🚀
Bryan K. Smith, chief of the Exploration Vehicle Project Office at NASA's John H. Glenn Research Center, provides the following explanation.
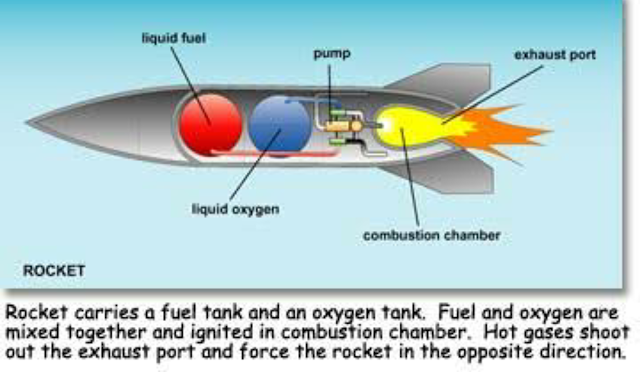
A rocket works by exchanging momentum. Both the mass of the propellant and the high velocity of its exit from the engine system give the rocket its momentum. The propellant attains its velocity by burning with an oxidizer in a high-pressure chamber. The resultant high energy exhaust is then funneled through a converging or diverging nozzle. This velocity, coupled with the right mass properties of the propellant, provides the power, or energy, required to get the vehicle into space.
Scientists measure the efficiency of rocket propellants by what is termed vehicle specific impulse. This measures the impulse, or change in momentum, per unit of propellant expended. Thrust, or the force generated by the propellant, is another critical property related to the density of the propellant. Unfortunately, propellants that have high specific impulse do not have high-density or high-thrust properties and vice versa.
Although high specific impulse is a desired quality while moving in space, propellants with high specific impulse will not create sufficient thrust to get into space from Earths surface. This is due to the larger fuel tanks necessary to contain a lower density propellant and the atmospheric drag that acts on the tanks when the rocket attempts to power beyond Earth's gravity. Other propellant considerations include ease of ignition, combustion stability, temperature, storability, reliability, toxicity, cost and availability. As a result, different propellants are used for different missions and differ among the stages of any given rocket.
Propellants can be in the form of a solid, liquid or gas, each with their own advantages and disadvantages. Solid propellants have higher density--and therefore thrust. They also are storable, transportable, reliable, less complex and can also contain their own oxidizer. Once ignited, however, solid propellants burn continuously, limiting the number of applications. Examples of rockets using solid propellants include the first stage of military missiles, commercial rockets and the first stage boosters that are attached to both sides of the liquid-fuel tank on the space shuttle. (Though attached, the two cylindrical boosters are separate units from the tank, which itself supplies the shuttle orbiters own liquid-fuel engines.) Ammonium perchlorate mixed with powdered aluminum that is held together in a rubberlike matrix is the most common solid propellant.
Liquids, in particular low temperature liquids, offer the highest specific impulse values and can be started and stopped at will throughout a mission, which makes them the best candidates for space travel. For example, liquid hydrogen and liquid oxygen have a very high specific impulse and are used for the upper or second stages of a rocket. Dense liquids such as RP-1--similar to kerosene--are sometimes used for the first stage but lack the high specific impulse for use in space. Rounding out the propellant options, gaseous fuels lack density but can offer some performance and long-term storage advantages for space travel.
The specific requirements of a mission are the primary consideration for propellant selection. For example, NASAs new human launch system, designed to replace the space shuttle, will use solid propellants for the first stage, liquid hydrogen and oxygen for the second stage, and liquid propellants for the service module in order to reach the International Space Station. This propellant architecture can then evolve to support future lunar and Mars missions.




Comments
Post a Comment